Dynamics at Surfaces
Theoretical understanding of surface chemistry will eventually become a tool to design new chemical technology including: heterogeneous (photo) catalysts, photovoltaics, fuel cells and much more. To reach this goal, we require new ideas and new theories of molecular interactions at interfaces. Applying cutting-edge laser, molecular beam, and ultrahigh vacuum technology to design well-defined experiments that can catch molecules in the act of reacting, our group strives to provide benchmark measurements which set standards for the next generation of theoretical advance. In particular, we seek to discover the "rules" that govern the conversion of energy at interfaces. Although too small to see with the naked eye and too fast to follow except with the fastest pulsed lasers, energy conversion takes place one molecule at a time and one collision at a time. By isolating these individual energy conversion events and studying them, we are building the conceptual bridge connecting our macroscopic experience of energy conversion to the molecular world.
Press Releases & Research News
With this award, the prize committee honors the Max Planck director’s research contributions to a better understanding of the dynamic interactions between molecules and surfaces.
more
Graphene is celebrated as an extraordinary material. It consists of pure carbon and is only a single layer of atoms thin. Nevertheless, it is extremely stable and even conductive. For electronics, however, graphene still has crucial disadvantages: It cannot be used as a semiconductor yet. Hydrogen atoms on graphene could change this. Researchers now showed how hydrogen atoms chemically bind to carbon atoms of graphene and what happens during this process.
more
When adsorbed at a salt crystal surface, the vibrations of excited carbon monoxide (CO) molecules are damped within milliseconds. This is due to electromagnetic interaction of the CO dipole with the salt crystal. In contrast, energy loss via the chemical bond is less important.
more
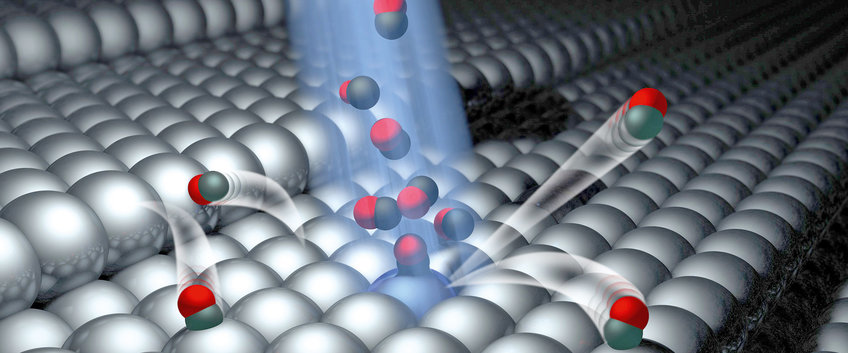
The Göttingen researchers Oliver Bünermann and Alec Wodtke of the MPI for Biophysical Chemistry and the University of Göttingen have made a big step forward in understanding chemical reactions at surfaces in detail. Their findings could, in future, help to further improve catalytic processes such as emission control and to identify new catalytic materials.
more
Göttingen researchers have now succeeded in generating ultra-short pulses of atoms. They might help to carry out time-resolved experiments initiated by atomic collisions.
more
Press Articles, Special Publications & Lectures